No products in the cart.
Medical Devices
Showing 1–30 of 104 results
- Medical Suction Accessories
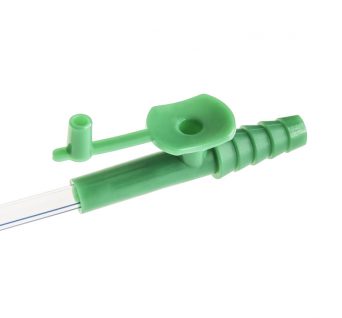
Adult Yankauer Suction Handle
For use with medical suction units
The Adult Yankauer suction tube is an indispensable part of a first responder’s tool-kit and a staple among hospital units.
It is used as an oral suctioning tool in medical procedures. It attaches directly to a pump and is ideal for first responders, first-aid environments and minor surgeries.
The firm plastic suction tip is disposable and features a large opening surrounded by a bulbous head.
SKU: 811030 - AN170002 - Nebuliser Accessories
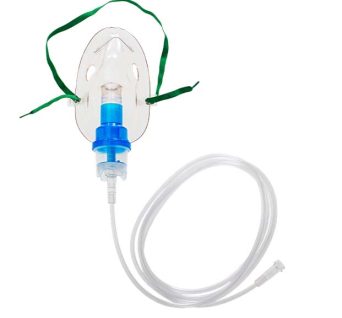
Nebuliser Mask, Adult
Designed to deliver medication in the form of a fine mist, or aerosol, directly to the lungs connected to compressor nebuliser.
The mask is connected to the air compressor, via the nebulizer bowl, by 2 meters of clear latex-free tubing attached with a swivel connection which permits some patient movement. This is typically used for patients with respiratory conditions such as asthma, COPD, bronchitis, or other respiratory infections that require direct lung treatment.
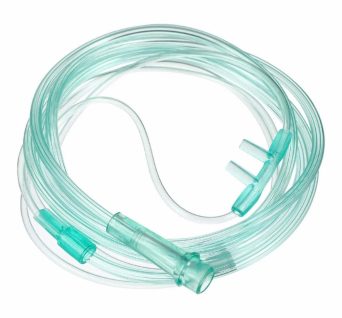
SKU: AN081001NS - Oxygen Therapy Consumables & Accessories
Oxygen Nasal Cannula, Adult Size
Super soft, flexible nasal prong inserts for added patient comfort. Kink resistant star lumen tubing
Nasal Oxygen Cannulas are made from non-toxic medical grade PVC and are designed to simplify oxygen administration and
are super soft and flexible to maximise patient comfort.For superior performance and patient satisfaction, choose our Nasal Oxygen Cannula with 2.1m clear tubing. It combines comfort, durability, and functionality to support effective oxygen therapy.
SKU: AN070002NS - Oxygen Therapy Consumables & Accessories
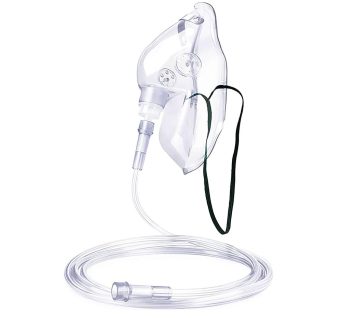
Oxygen Mask With Tubing
With elastic straps, adjustable aluminum nose clip to ensure secure and comfortable fit. Made latex free from medical grade PVC.
For delivering effective aerosol and oxygen therapy, the Adult-sized oxygen mask with Tubing allows for needed medications, oxygen, or even humidity to be placed in a patient’s respiratory tract and lungs.
Perfect for hospitals, clinics, and home care. Ensure optimal oxygen delivery with a mask that prioritizes patient comfort and clinical effectiveness. Order now to stock up on this essential medical supply!
SKU: AN061003NS X - Suction Machines
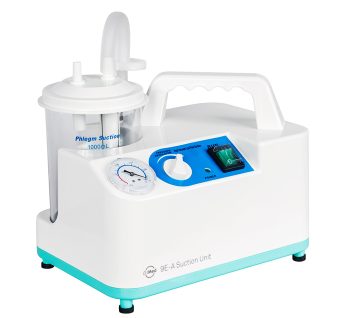
9E-A Medical Suction Unit
Medical suction machine to remove phlegm and mucus fluids.
This medical suction pump unit is used to remove fluids like mucus, blood, saliva, or phlegm that are causing obstructions on the airway or respiratory system and infectious materials from wounds.
9E-A suction unit can cater to both adult and pediatric patients.
- 20L/min suction rate
- AC Mains Power
Order now for a FREE delivery!
SKU: 810001 - Sphygmomanometers
Aneroid Sphygmomanometer Two Handed
Aneroid sphygmomanometer consists of an inflatable cuff, a measuring unit and bulb and valve
Used to measure blood pressure, composed of an inflatable cuff to collapse and then release the artery under the cuff in a controlled manner and an aneroid manometer to measure the pressure
The simple, classic design is easy to use and has been relied upon by healthcare professionals for many years.
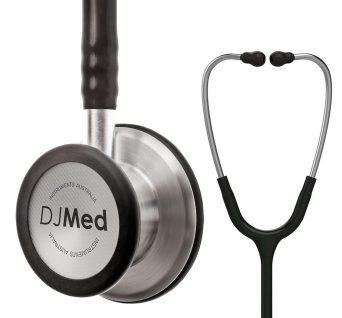
SKU: 5189 - Stethoscopes
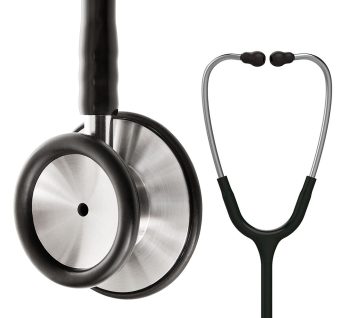
Diagnostic Stethoscope, Dual Head
Classic dual head, a bell and floating, tunable diaphragm design, made with precision stainless steel
Dual head stethoscope is a quality diagnostic instrument used for the auscultation of heart, lung and bowel sounds.
Hard-working clinical tool with the acoustic sensitivity and versatility you can rely on.
SKU: 505150 - Nebuliser Accessories
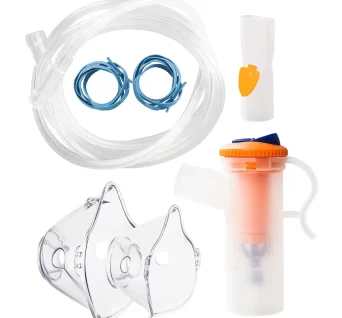
Nebuliser Bowl Kit
Nebuliser kit with masks, nebuliser bottle and tubbing.
An accessory set, which includes masks, bottles and tubings for those patients undergoing nebuliser therapy at home. It comes in with two sizes for the mask, one for adult use and another for pediatric use.
Masks are adjustable to provide for a perfect fit for the user.
SKU: 911021 - Pulse Oximeters
Pulse Oximeter, Blood Oxygen Monitor
Meet One of the World’s Most Advanced Pulse Oximeters
Pulse Oximeter measures your Oxygen Saturation (Sp02) & Pulse Rate.
The oximeter is portable and ideal for monitoring respiratory conditions; pre/post-exercise and pre/post-operative conditions.
SKU: 200201 - Nebulisers
Homecare Compressor Nebuliser
Stylish compressor nebuliser perfect for facility and homecare use
DJMed homecare nebuliser from DJMed is built to treat a range of respiratory conditions: COPD, asthma, and other lung diseases.
This device produces a fine mist of droplets that contain active medication delivered into the lungs to help your condition.
The nebuliser is small, strong, and simple to use. With proper use and care, this device will provide you with many years of treatment.
Order now for a FREE delivery!
SKU: 9100023 - Nebulisers
Clinical Compressor Nebuliser
Clinical compressor nebuliser with accessory storage compartment
For patients with a respiratory illness, such as asthma, nebulisers offer a quick and effective way to find relief from their symptoms.
With the use of a nebulizer, patients can inhale their prescribed medication directly into the lungs, giving them fast relief from inflammation — and allowing them to breathe easier.
SKU: 910024 - Stethoscopes
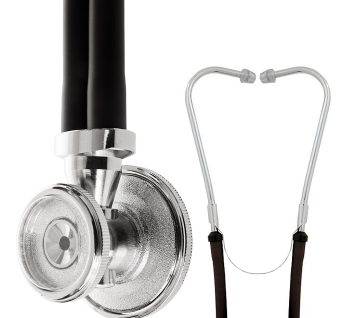
Sprague Rappaport Stethoscope
Affordable, multi-purpose stethoscope with excellent acoustics designed for clinical use.
The legendary design features of the Sprague have made it one of the most popular stethoscopes in history, traditionally trusted to detect faint heart sounds and murmurs.
Unique five-in-one stethoscope that eliminates the need for different stethoscopes for infants, children, and adults.
SKU: 3523 - Oxygen Therapy Consumables & Accessories
Nasal Oxygen Cannula Ear Cushions
Tube cushions are soft, closed-cell foam cushions that slide over the head or face tube of an oxygen cannula
This will provide a cushion to reduce chafing or pressure on the ear area. It is Latex-free. Sold as a pair (2pcs)
SKU: 1016-0 - Thermometers
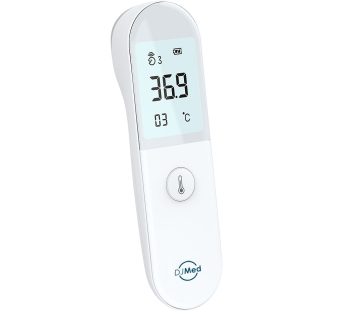
DJMed T2 Infrared Thermometer
Non-contact digital infrared thermometer suitable for all ages and surfaces.
DJMed T2 is a non-contact digital thermometer using a non-contact infrared to measure body temperature from the temple for adult and child at home.
This thermometer can also measure surface temperature.
SKU: 200102 - TENS Machines
Digital Therapy, Rechargeable TENS Machine
Dual channel, digital electrotherapy pain relief EMS & TENS unit with USB rechargeable lithium battery.
Digital TENS Machine is an easy to use machine that offers non-invasive, natural pain relief without the use of any drugs or chemicals.
SKU: 300051 - Oxygen Concentrators
DJMed 5L Oxygen Concentrator
Low maintenance, easy-to-use, low noise advanced digital 5L @ 93% Oxygen Concentrator.
Small profile fits easily into home, easy-access humidifier with simple to read controls and alarms. When you need a constant steady, and reliable flow of oxygen you need DJMed’s continuous flow technology.
Set your device to the litter by minute prescribed by your doctor and rest assured that you are receiving an uninterrupted stream of oxygen.
Order now for a FREE delivery!
SKU: 850000 - Stethoscopes
Classic Stethoscope
Hard-working, dependable and sensitive Classic stethoscope is perfect for assessing children or adults
Tunable, adjustable frequency, floating diaphragm allows tuning to enhance high- and low-frequency response in both the adult and pediatric diaphragms.
Flexible PVC tubing provides excellent sound conduction, and the sleek design of this product offers professional styling along with superior clinical performance.
SKU: n/a - TENS & EMS Accessories
TENS Electrode Pads, Square, 50x50mm, White
Self-adhesive, re-usable, long Life, 50×50 mm, 2mm plug, 10pcs pads included
Reusable electrodes with white super soft cotton (allow attachment to skin smoothly) backing and premium adhesive gel.
Popular size, bio-compatible, long life dry out Resisting, durable, comfortable pads that gently adhere to your skin. Perfect for TENS and EMS.
SKU: 300501 - TENS Machines
Pro Therapy TENS Machine
Dual channel, professional high frequency, wide pulse width max. intensity TENS unit with a safe-storage case.
TENS machine offers a proven and effective pain relief together with a portable travel size. TENS machine offers program modes that are preset for relaxing variable massage and various electronic pulse modes to help relieve acute and chronic pain.
TENS machine is easy to use with pre-set automatic programs for suit parts of your body such as back, shoulders and more. With an easy visual body icon on the LCD display that displays massage style, intensity and time remaining.
SKU: 300050 - TENS & EMS Accessories
TENS Electrode Pads, Square, 50x50mm, Black
Self-adhesive, re-usable, long Life, 50×50 mm, 2mm plug, 10pcs pads included
Reusable electrodes with black super soft cotton (allow attachment to skin smoothly) backing and premium adhesive gel.
Popular size, bio-compatible, long life dry out Resisting, durable, comfortable pads that gently adhere to your skin. Perfect for TENS and EMS.
SKU: 300502 - Nursing Alarms & Pagers
Two Call Buttons and Pager Kit for the Elderly
2 x Call Buttons – Mobile Pager – Easy One Button Use – 50m Call Distance
Caregiver Wireless Pager, a Nurse Call System
A Caregiver wireless pager comes with two buttons, one could be placed in the bathroom and one to hang around your neck, upstairs and one down stairs or wherever one may wish.
Wireless nurse call system comes with a mobile pager for the carer to carry in their pocket or to clip onto their belt. With one simple press of a button the resident is able to call the carer for assistance.
The wireless pager has a range is significant at 50m allowing the caregiver freedom of movement and the resident a feeling of safety.
SKU: 2014 - Oxygen Therapy Consumables & Accessories
6m Adult Nasal Cannula, Kink Resistant
High flow, kink resistant with convenient, extra long 6m tubbing oxygen nasal cannula.
The 6m Adult Nasal Cannula features soft, non-irritating nasal prongs for patient comfort. It is kink resistant and has a convenient extra-long 6m tubing for added flexibility and comfort.
The soft latex-free material is gentle on the skin and helps reduce irritation and soreness. Most importantly, this cannula has a high delivery rate and is designed for a large range of oxygen
SKU: 850108
Medical Devices
In Australia, a medical device is defined and regulated by the Therapeutic Goods Administration (TGA), which is the regulatory authority responsible for ensuring the safety and efficacy of therapeutic goods, including medical devices. The TGA defines a medical device as follows:
"A medical device is any instrument, apparatus, appliance, software, material, or other article intended to be used for a medical purpose and does not achieve its primary intended action by pharmacological, immunological, or metabolic means in or on the human body, but which may be assisted in its intended function by such means."
This definition encompasses a wide range of products used in healthcare, including but not limited to:
Diagnostic Equipment: Such as blood glucose monitors, X-ray machines, and MRI scanners. Surgical Instruments: Such as scalpels, forceps, and surgical implants like joint replacements or pacemakers. Medical Implants: Devices that are surgically placed inside the body, such as artificial joints, heart valves, or stents. Diagnostic Software: Software used for medical diagnosis, monitoring, or treatment planning. Personal Protective Equipment (PPE): Certain medical devices used for infection control and safety, including surgical masks, gloves, and face shields. In vitro Diagnostic (IVD) Devices: Devices used for testing samples outside the body, such as pregnancy tests, blood glucose test strips, and laboratory equipment. Assistive Devices: Products designed to assist individuals with disabilities or medical conditions, such as hearing aids, wheelchairs, and walking aids. Dental Equipment: Dental instruments, X-ray machines, and dental implants are also considered medical devices. Wound Care Products: Dressings, bandages, and wound management devices.
Medical devices in Australia are classified into various risk classes (Class I, Class IIa, Class IIb, Class III, and Active Implantable Medical Devices), and the level of regulatory scrutiny and requirements vary depending on the classification. The TGA assesses and regulates medical devices to ensure they meet safety, quality, and performance standards before they can be legally marketed and used in Australia.
Manufacturers of medical devices are required to comply with regulatory requirements, including registering their products with the TGA and maintaining appropriate records. Healthcare professionals and consumers can also access information about registered medical devices on the TGA's public database.
It's important to note that medical devices play a critical role in healthcare, and their regulation is essential to ensure patient safety and the effectiveness of these products. If you have questions about a specific medical device or its regulatory status in Australia, it is advisable to consult with healthcare professionals or refer to the TGA's official resources.
What is an example of a medical device?
Certainly, here are some examples of medical devices:
Blood Pressure Monitor: This device is used to measure a patient's blood pressure and is often used at home or in clinical settings. Thermometer: A common medical device used to measure body temperature, which is essential for diagnosing fever and monitoring a patient's health. Insulin Pump: Used by people with diabetes, an insulin pump delivers a controlled dose of insulin to regulate blood sugar levels. Pacemaker: An implantable medical device used to regulate the heart's rhythm by sending electrical impulses to the heart muscles. Ventilator: A machine that assists patients with breathing when they are unable to do so adequately on their own, often used in intensive care units. Infusion Pump: Used to deliver fluids, medications, or nutrients directly into a patient's bloodstream in a controlled manner. X-ray Machine: A medical imaging device that uses X-rays to create images of the body's internal structures, helping diagnose and monitor various conditions. Blood Glucose Monitor: A device used by people with diabetes to measure their blood glucose levels and manage their condition. EKG/ECG Machine: A device that records the electrical activity of the heart, commonly used to diagnose heart conditions. Ultrasound Machine: Medical imaging equipment that uses sound waves to create real-time images of internal organs and tissues.
These examples illustrate the diversity of medical devices and their crucial roles in healthcare, from diagnostic tools to therapeutic and life-supporting devices. The classification and regulation of medical devices vary by country and region to ensure their safety, effectiveness, and quality of use in healthcare settings.